Carbon nanodots (CNDs) are tiny, light-emitting nanoparticles that have fascinated researchers for their versatility in imaging, catalysis, and nanomedicine. Unveiling the true molecular identity of CNDs has long been a challenge: these tiny, luminescent nanoparticles are easy to make, but difficult to define with precision.
Our recent study, now published in Angewandte Chemie International Edition, takes a decisive step in addressing this challenge. The work was carried out in collaboration with the group of Prof. Maurizio Prato (University of Trieste) and Dr. Serena Agostini from Malvern Panalytical (UK). The research was supported by the European Research Council (ERC AdG-2019 n. 885323 – e-DOTS; ERC StG n. 101039578 – PROTOMAT), the Italian Ministry of University MIUR (cofin Prot. 20228YFRNL), with additional support from FRA2024 (University of Trieste) and Microgrants 2024 funded by Region FVG (LR 2/2011, Art. 4).
To understand how CNDs are born and evolve, we first explored the early intermediates formed during the hydrothermal synthesis of L-arginine and ethylenediamine. Using advanced chromatographic and spectroscopic tools, we were able to isolate and confirm the structures of elusive molecular species that appear in the first stages of the reaction, fleeting building blocks that ultimately collapse and reorganize into CNDs. These insights enrich our mechanistic picture, but they also served as a crucial prelude to the main challenge: quantifying the molecular identity of the final nanomaterials.
By employing multi-detection gel permeation chromatography, we established the absolute molecular weight of CNDs (around 4,400 g mol⁻¹ on average) together with a narrow dispersity, a result never achieved before in this field with this modern and advanced technology. Complementary analyses confirmed their reproducible size of just a few nanometers, while colorimetric and spectroscopic assays revealed that each CND carries approximately seven reactive amine groups on its surface.
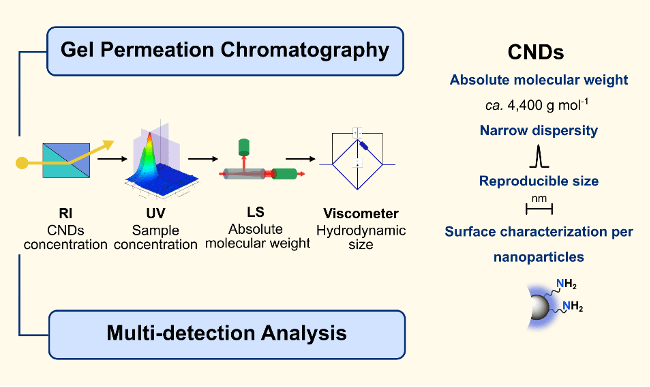
This combination of precise size, weight, and surface chemistry transforms CNDs from mysterious nanoparticles into well-defined, tunable nanoplatforms. More than numbers, these results provide a reliable framework for designing CNDs, paving the way for their use as versatile functional materials in next-generation technologies and hybrid nanomaterials.
Link to the open access article.


